Here We Go....(NASDAQ:VRPX)
Good morning HUMANS!!
We are closing this week on a HIGH Note. **NASDAQ:VRPX** Has been ON FIRE since the market closed yesterday… Can you imagine what happens when the opening bell strikes?
Keep in mind today is going to be WILD. Full of excitement and most likely full of swings. There will be PLENTY OF opportunities to make some sweet moolah so keep your eyes glued to this little beauty ALL DAY!
Biotech companies have a history of going from Zero to HERO or vice versa. Remember that most drugs require FDA approval. During clinical trials drug companies often rally, pull back and everything in between until they reach the ultimate goal, which is that Golden stamp from the FDA giving it the approval!
So as we close this week out ENJOY!!
**VRPX** Is sure to keep your excitement at an all time high today!!!
(NASDAQ:VRPX) TO The MOON!!!
Full report below
Virpax Pharmaceuticals, Inc. (Nasdaq: VRPX) announced on Thursday the completion of a full study that followed the initial Probudur™ pilot study performed by the U.S. Army Institute of Surgical Research (USAISR) under an existing Cooperative Research and Development Agreement (CRADA).
Who is the USAISR?
The USAISR is the U.S. Department of Defense's premier research organization for developing solutions for trauma and critical care challenges in combat casualties.
What was the point of the study?
The study was designed to determine if Probudur reduces pain behaviors in a rat model of incisional pain. The surgical procedures and assessments were identical to those in the pilot study. The study compared Probudur with free bupivacaine and EXPAREL®.
What was done?
Various concentrations of Probudur were injected into the tissue around the incision site as well as a saline solution for the control group.
What was shown?
The doses of Probudur showed reduction in incision-induced pain behaviors. "These positive results are consistent with what we at Virpax have previously observed and we are encouraged by these findings," commented Jatinder Dhaliwal, Chief Executive Officer of Virpax.
Probudur is being developed to achieve the overall goal of safe and effective pain control during the perioperative period and to significantly reduce or eliminate the need for opioids after surgery in approved indications.
Discover the company that Forbes is calling a *Promising Stock* in a sickly biotech market!

** Nasdaq: VRPX **
OVERVIEW
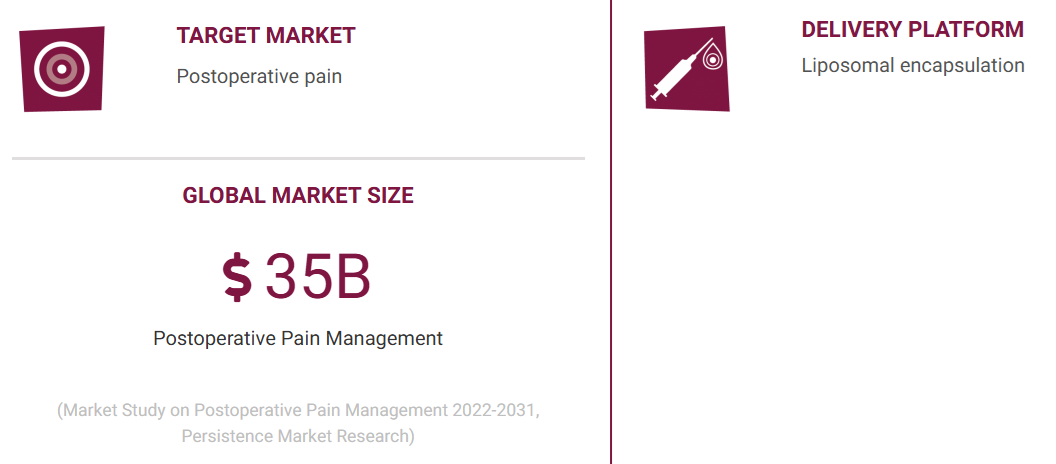
Virpax Pharmaceuticals, Inc. (NASDAQ: VRPX) is combatting the expensive and deadly opioid epidemic by developing non-addictive products for pain management using its proprietary technologies to optimize and target drug delivery.
The company is seeking FDA approval in three multi-billion-dollar markets. Probudur for postoperative pain, Envelta for severe pain including post cancer pain, and NobrXiol for the management of rare pediatric epilepsy and potential orphan diseases.
If approved, these product candidates will fundamentally change what prescribers recommend managing their patients' severe pain, Epilepsy, and other CNS disorders in the future.
Understanding the Opioid Overdose Epidemic
What to know:
- The number of opioid-involved deaths has increased substantially since 1999.
- There have been three distinct waves of increases in opioid overdose deaths over the last 25 years, with each wave driven by different types of opioids.
- The U.S. Congress Joint Economic Committee (JEC)—led by Chairman Don Beyer (D-VA)—released a new analysis that finds the opioid epidemic cost the United States a record of nearly $1.5 trillion in 2020. This is up a staggering 37% from 2017, when the CDC last measured the cost.
A Competitive Edge with Probudur
Virpax's pain management drug candidate and lead product candidate, Probudur, has what's known as "stickiness," the company says, meaning that in preclinical studies it lasts longer than rivals' products.
Probudur is a local anesthetic that binds to the sodium channel, preventing pain signals from reaching the brain. In preclinical studies, Probudur has shown long duration pain control for at least 96 hours, with a rat incisional model demonstrating analgesia for up to five days and in vitro studies demonstrating a slow release of bupivacaine that lasted for up to six days.
POTENTIAL BENEFITS
- Animal studies demonstrated 96 hours of local anesthetic activity at wound site
- May eliminate the need for opioids after surgery!
- May reduce associated costs and length of hospital stay
- Produced no evidence of motor or sensory nerve damage at a dose that was 10 times higher than free bupivacaine in animal studies
NEW DRUG APPLICATION (NDA)
As a result of the company's PreIND review, the FDA has indicated that it is reasonable for Virpax to pursue an accelerated 505(b)(2) New Drug Application for Probudur.
Study Results
A minipig Dose Range Finding ("DRF") study for Probudur was conducted to evaluate the tolerance of Probudur in an incisional wound healing model in minipigs. Probudur was injected locally into the tissue surrounding the incision area. All of the minipigs demonstrated positive tolerance to Probudur and no adverse effects were noted. The development program for Probudur continues to support the Company's belief that Probudur has the potential to provide both immediate and sustained pain relief at the incisional area.
"These positive study results in our pharmacokinetics and safety studies for Probudur and continue to demonstrate both immediate relief as well as sustained relief at the wound site," stated Jatinder Dhaliwal, Chief Executive Officer of Virpax. "The completion of these studies brings us another step closer to filing our Investigational New Drug Application (IND) for Probudur."
Military grants and CRADA awards for VRPX's Rx product candidates
- US Army Institute of Surgical Research (USAISR)
Awarded 5/05/2022
Extended 10/09/2023 - National Center for Advancing Translational Sciences (NCATS)
Awarded 8/31/2020 - National Institute of Neurological Disorders and Stroke (NINDS)
Awarded 4/19/2023
Targeting the Brain to Manage CNS Disorders
The company's patented Molecular Envelope Technology (MET) platform is being used to grow our Central Nervous System (CNS) disorder pipeline. The current CNS product indications include Post Traumatic Stress Disorder (PES200), and Rare Pediatric Epilepsy (NobrXiol).
MET will significantly improve our drug delivery process characteristics by directly targeting the brain to manage CNS disorder symptoms.
Virpax is collaborating with the National Advisory Neurological Disorders and Stroke Council (NINDS) and Epilepsy Therapy Screening Program (ETSP) to begin preclinical development of NobrXiol for the treatment of pediatric epilepsy. The mission of ETSP is to identify novel agents that may address unmet medical needs in epilepsy.
What is Forbes Saying?
"Few investors have the scientific background to assess whether a fledgling biotech firm has the stuff to go on to glory. But some signals point to which could be the most promising, such as grants they have received and high-level outside interest. An example of that is Virpax Pharmaceuticals, which focuses on pain management—and does so without the addictive aspect of opioids. The company has received grants from the National Institutes of Health and the Pentagon. The U.S. Army is studying the use of one of its drugs for battlefield wounds and other injuries."
In Summary
VRPX is addressing the opioid crisis and lucrative markets by delivering innovative and safer pain management solutions. To see all of the company's products, click HERE.
Biopharma companies are tied closely to study data and approval decisions from the U.S. Food and Drug Administration or other drug regulators.
Keep a close eye on VRPX's news for any potential progress with the FDA! Promising news could serve as a tremendous catalyst for growth potential.